

Consuming CBD wine may have potential health risks, especially when it comes to its impact on liver function. The liver plays a crucial role in metabolizing substances that enter the body, including alcohol and other chemicals. When CBD is consumed in wine form, it can put added stress on the liver as it works to break down both the CBD and the alcohol present in the wine.
Research has shown that high doses of CBD can cause liver toxicity in some individuals, particularly those with pre-existing liver conditions or those taking medications that affect liver function. Adding alcohol into the mix further complicates matters, as excessive alcohol consumption is already known to be harmful to the liver.
While more research is needed to fully understand the effects of consuming CBD wine on liver function, it is important for individuals to be aware of the potential risks involved. It is recommended to consume CBD wine in moderation and consult with a healthcare provider before incorporating it into your routine, especially if you have any underlying health concerns related to liver function. Prioritizing your overall health and well-being should always be a top priority when considering any new dietary or lifestyle choices.
CBD wine has gained popularity in recent years as a trendy way to enjoy the benefits of both CBD and alcohol. However, it is important to consider the potential health risks associated with consuming this unique beverage. One major concern is the potential for drug interactions.
CBD, short for cannabidiol, is a compound found in cannabis plants that has been touted for its therapeutic effects. It interacts with the body's endocannabinoid system, which plays a role in regulating various functions such as mood, appetite, and pain sensation. When CBD is consumed alongside other medications, there is a risk of interactions that could potentially cause harm.
For example, CBD has been shown to inhibit certain enzymes in the liver that are responsible for metabolizing drugs. This means that if someone is taking medication that is broken down by these enzymes, the presence of CBD could lead to higher levels of the drug in the bloodstream. This can increase the risk of side effects or toxicity.
In addition, some medications may also affect how the body processes CBD, leading to potential complications when consumed together. It is always important to consult with a healthcare provider before combining CBD wine with any other medications to ensure safety and minimize risks.
While CBD wine may offer a unique way to enjoy the benefits of CBD while indulging in a glass of wine, it is crucial to be aware of the potential for drug interactions. By being informed and cautious about mixing substances, you can enjoy your CBD wine responsibly and minimize any potential health risks.
When it comes to consuming CBD wine, one potential health risk to consider is the risk of addiction or dependence. While CBD on its own is not considered addictive, when combined with alcohol in a wine form, there may be an increased likelihood of developing a dependence on the substance.
CBD has been shown to have calming and relaxing effects, which can make it appealing for those looking to unwind after a long day. When combined with the effects of alcohol, which is also known for its ability to reduce stress and anxiety, the combination may create a heightened sense of relaxation that some individuals may seek out regularly.
Over time, this repeated use of CBD wine to achieve a desired level of relaxation can lead to tolerance, where larger amounts are needed to achieve the same effects. This can then progress to dependence, where the individual feels like they need CBD wine in order to function normally or cope with everyday stressors.
Additionally, excessive consumption of alcohol in any form can lead to physical dependence and addiction, which can further compound the risks associated with consuming CBD wine. It's important for individuals considering using CBD wine to be mindful of their consumption habits and seek help if they feel like they may be developing a dependency on the substance.
In conclusion, while CBD wine may offer potential health benefits when consumed in moderation, there is a risk of addiction or dependence when used excessively. It's important for individuals to be aware of these risks and approach consuming CBD wine with caution.
Consuming CBD wine has been a topic of interest for many, as it combines the potential health benefits of CBD with the enjoyment of a glass of wine. However, it is important to consider the effects that this combination may have on mental health.
CBD, or cannabidiol, is a compound found in cannabis that has been shown to have various therapeutic effects, including reducing anxiety and improving mood. When combined with alcohol, such as in CBD wine, these effects may be enhanced or altered.
While some people may find that consuming CBD wine helps them relax and unwind, others may experience negative effects on their mental health. Alcohol is a depressant that can worsen symptoms of anxiety and depression, and combining it with CBD may amplify these effects.
Additionally, there is still much research to be done on the long-term effects of consuming CBD wine on mental health. It is possible that regular consumption could lead to dependence or other negative outcomes.
Overall, while consuming CBD wine may have some potential health benefits, it is important to consider the impact it may have on mental health. It is always best to consult with a healthcare professional before incorporating any new substances into your routine.
 |
||
 |
||
| Clinical data | ||
|---|---|---|
| Pronunciation | /kæ.nÉ™.bÉ™.ˈdaɪ.É™l/ | |
| Trade names | Epidiolex, Epidyolex | |
| Other names | CBD, cannabidiolum, (−)-cannabidiol[1] | |
| AHFS/Drugs.com | Monograph | |
| MedlinePlus | a618051 | |
| License data |
|
|
| Pregnancy category |
||
| Addiction liability |
None [3] | |
| Routes of administration |
By mouth,[4] sublingual, buccal,[5]inhalation | |
| Drug class | Cannabinoid | |
| ATC code | ||
| Legal status | ||
| Legal status | ||
| Pharmacokinetic data | ||
| Bioavailability | Sublingual: 12–35%[13] | |
| Elimination half-life | 18–32 hours[14] | |
| Identifiers | ||
|
||
| CAS Number | ||
| PubChem CID | ||
| IUPHAR/BPS | ||
| DrugBank | ||
| ChemSpider | ||
| UNII | ||
| KEGG | ||
| ChEBI | ||
| PDB ligand | ||
| CompTox Dashboard (EPA) | ||
| ECHA InfoCard | 100.215.986 | |
| Chemical and physical data | ||
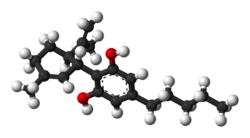
| Formula | C21H30O2 | |
| Molar mass | 314.469 g·mol−1 | |
| 3D model (JSmol) | ||
| Melting point | 66 °C (151 °F) | |
| Boiling point | 160–180 °C (320–356 °F) [15][unreliable medical source?] | |
| Solubility in water | Insoluble | |
|
||
|
||
| (verify) | ||
| Part of a series on |
| Cannabis |
|---|
 |
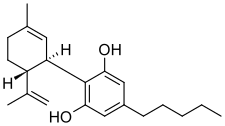
Cannabidiol (CBD) is a phytocannabinoid, one of 113 identified cannabinoids in cannabis plants, along with tetrahydrocannabinol (THC), and accounts for up to 40% of the plant's extract.[16] Medically, it is an anticonvulsant used to treat multiple forms of epilepsy.[4] It was discovered in 1940 and, as of 2024 clinical research on CBD included studies related to the treatment of anxiety, addiction, psychosis, movement disorders, and pain, but there is insufficient high-quality evidence that CBD is effective for these conditions.[17][18][19][20] CBD is sold as an herbal dietary supplement and promoted with yet unproven claims of particular therapeutic effects.[21]
Cannabidiol can be taken internally in multiple ways, including by inhaling cannabis smoke or vapor, swallowing it by mouth, and through use of an aerosol spray into the cheek.[22][23] It may be supplied as CBD oil containing only CBD as the active ingredient (excluding THC or terpenes), CBD-dominant hemp extract oil, capsules, dried cannabis, or prescription liquid solution.[4][19] CBD does not have the same psychoactivity as THC,[24][25] and can modulate the psychoactive effects of THC on the body if both are present.[16][24][26][27] Conversion of CBD to THC can occur when CBD is heated to temperatures between 250–300 °C, potentially leading to its partial transformation into THC.[28]
In the United States, the cannabidiol drug Epidiolex was approved by the Food and Drug Administration (FDA) in 2018 for the treatment of two seizure disorders.[4] While the 2018 United States Farm Bill removed hemp and hemp extracts (including CBD) from the Controlled Substances Act, the marketing and sale of CBD formulations for medical use or as an ingredient in dietary supplements or manufactured foods remains illegal under FDA regulation, as of 2024[update].[29][30]
In the United States, the FDA has indicated only one brand of prescription cannabidiol called Epidiolex for the treatment of seizures associated with Dravet syndrome, Lennox–Gastaut syndrome, or tuberous sclerosis complex in people one year of age and older.[4][31][32][33] While Epidiolex treatment is generally well tolerated, it is associated with minor adverse effects, such as gastrointestinal upset, decreased appetite, lethargy, sleepiness, and poor sleep quality.[4][32][34][33]
In the European Union, Epidyolex is indicated for use as adjunctive therapy of seizures associated with Lennox–Gastaut syndrome or Dravet syndrome, in conjunction with clobazam, for people two years of age and older.[10] In 2020, the label for Epidiolex in the US was expanded to include seizures associated with tuberous sclerosis complex. Epidiolex/Epidyolex is the first prescription formulation of plant-derived cannabidiol approved by regulatory bodies in the US and Europe.[35]
Research on other uses for cannabidiol includes several neurological disorders, but the findings have not been confirmed to establish such uses in clinical practice.[14][18][19][24][36][37][38] In October 2019, the FDA issued an advisory warning that the effects of CBD during pregnancy or breastfeeding are unknown, indicating that the safety, doses, interactions with other drugs or foods, and side effects of CBD are not clinically defined, and may pose a risk to the mother and infant.[39]
Many claims are made for the therapeutic benefit of cannabidiol that are not backed by sound evidence. Some claims, such as treatment of cancer, are pseudoscience.[21]
In 2020, the label for Epidiolex in the US was expanded to include treatment of seizures associated with tuberous sclerosis.[31]
Acclaimed for relieving chronic pain, some researchers conclude that the evidence is insufficient to determine the effectiveness of CBD in pain relief, primarily due to the challenging access to pure CBD.[40]
CBD oil is used for massage therapy as a substitute for body oil and for its health benefits.[41]
Cannabidiol does not appear to have any intoxicating effects[42] such as those caused by ∆9-THC in cannabis, but it is under preliminary research for its possible anxiolytic and antipsychotic effects.[18][19][25] As the legal landscape and understanding about the differences in medical cannabinoids unfolds, experts are working to distinguish "medical cannabis" (with varying degrees of psychotropic effects and deficits in executive function) from "medical CBD therapies", which would commonly present as having a reduced or non-psychoactive side-effect profile.[19][25][43]
Various strains of "medical cannabis" are found to have a significant variation in the ratios of CBD-to-THC and are known to contain other non-psychotropic cannabinoids.[44] Any psychoactive cannabis, regardless of its CBD content, is derived from the flower (or bud) of the genus Cannabis. As defined by US federal law, non-psychoactive hemp (also commonly termed "industrial hemp"), regardless of its CBD content, is any part of the cannabis plant, whether growing or not, containing a ∆9-tetrahydrocannabinol concentration of no more than 0.3% on a dry-weight basis.[45] In the United States, certain standards are required for legal growing, cultivating, and producing the hemp plant, but there are no federal standards for quality being enforced in the hemp industry. Certain state regulations are in place, but vary state to state.[46] For instance, the Colorado Industrial Hemp Program registers growers of industrial hemp and samples crops to verify that the dry-weight THC concentration does not exceed 0.3%.[45]
CBD is present as an active constituent of cannabis, which is used both recreationally and medically.
Nabiximols (brand name Sativex), an oromucosal spray made of a complex botanical mixture containing cannabidiol (CBD), delta-9-tetrahydrocannabinol (THC), and additional cannabinoid and non-cannabinoid constituents from cannabis sativa plants, was approved by Health Canada in 2005, to treat central neuropathic pain in multiple sclerosis, and in 2007, for cancer-related pain.[47] In New Zealand, Sativex is "approved for use as an add-on treatment for symptom improvement in people with moderate to severe spasticity due to multiple sclerosis who have not responded adequately to other anti-spasticity medication."[48]
Epidiolex (Epidyolex in Europe) is an orally administered cannabidiol solution.[4][10] It was approved in 2018 for treatment of two rare forms of childhood epilepsy, Lennox–Gastaut syndrome and Dravet syndrome, and seizures associated with tuberous sclerosis complex.[4][10] In the US, it is approved in these indications for people one year of age and older.[4]
Research indicates that cannabidiol may reduce adverse effects of THC, particularly those causing intoxication and sedation, but only at high doses.[49] Safety studies of cannabidiol showed it is well tolerated, but may cause fatigue, somnolence, sedation, diarrhea, or changes in appetite as common adverse effects, with the most common being somnolence and sedation. Side effects of CBD are dose related.[50] Epidiolex documentation lists sleepiness, insomnia and poor quality sleep, decreased appetite, diarrhea, and fatigue.[4][51]
In November 2019, the FDA issued concerns about the safety of cannabidiol, stating that CBD use has potential to cause hepatotoxicity, interfere with the mechanisms of prescription drugs, produce gastrointestinal disorders, or affect alertness and mood.[52] Over 2020–23, the FDA updated its safety concerns about CBD,[53] acknowledging the unknown effects of protracted use, how it affects the developing brain, fetus, or infants during breastfeeding, whether it interacts with dietary supplements or prescription drugs, whether male fertility is affected, and its possible side effects, such as drowsiness.[54]
As of September 2019[update], 1,085 people contacted US poison control centers about CBD-induced illnesses, doubling the number of cases over the 2018 rate and increasing by 9 times the case numbers of 2017.[55] Of cases reported in 2019, more than 33% received medical attention and 46 people were admitted to a hospital intensive care unit, possibly due to exposure to other products, or drug interactions with CBD.[56]
In 2022, the FDA stated that "scientific studies show possible harm to the male reproductive system, including testicular atrophy, harm to the liver, and interactions with certain medications. The FDA has not found adequate information showing how much CBD can be consumed, and for how long, before causing harm. This is particularly true for vulnerable populations like children and those who are pregnant."[57]
Laboratory evidence indicated that cannabidiol may reduce THC clearance, increasing plasma concentrations which may raise THC availability to receptors and enhance its effect in a dose-dependent manner.[58][59] In vitro, cannabidiol inhibited the activity of voltage-dependent sodium and potassium channels, which may affect neural activity.[60] A recent study using X-ray crystallography showed that CBD binds inside the sodium channel pore at a novel site at the interface of the fenestrations and the central hydrophobic cavity of the channel. Binding at this site blocks the transmembrane-spanning sodium ion translocation pathway, providing a molecular mechanism for channel inhibition, which could contribute to a reduced excitability.[61] A small clinical trial reported that CBD partially inhibited the CYP2C-catalyzed hydroxylation of THC to 11-OH-THC.[62] Little is known about potential drug interactions, but CBD mediates a decrease in clobazam metabolism.[63] Work with human liver microsomes shows that cannabidiol inhibits CYP3A5 and CYP3A4 to some degree.[64]
In vitro, cannabidiol has low affinity for, and acts as a negative allosteric modulator of the CB1 cannabinoid receptor[65][66]
Cannabidiol may be an antagonist of GPR55, a G protein-coupled receptor and putative non-homologous CB3 cannabinoid receptor shown by in vitro studies to be widely distributed in the brain.[67][68][69] Cannabidiol may interact with various neurotransmitters, such as serotonin, dopamine, and GABA.[67][68][70]
As of 2024, the cellular effects and mechanisms of cannabidiol in vivo are unknown,[4][10] as research to date has been inconclusive and based on laboratory studies.[67] The anticonvulsant effects provided by cannabidiol (Epidiolex) in people with certain forms of epilepsy do not appear to involve cannabinoid receptors.[4] A possible mechanism for the effects of cannabidiol on seizures is by affecting the neuronal movement of calcium in brain structures involved in the excessive electrical activity of seizures.[10]
The oral bioavailability of cannabidiol is approximately 6% in fasting state and 36.5—57.3% in fed-state[71] in humans, while its bioavailability via inhalation is 11 to 45% (mean 31%).[11][12][71][72] The oral bioavailability of cannabidiol varies based on several factors such as formulation,[72] dose, and food intake.[71] The sublingual bioavailability of cannabidiol is approximately 12 to 35%.[73] The elimination half-life of cannabidiol in blood is 56 to 61 hours after oral doses twice per day over 7 days.[4] Based on the pharmacokinetic analysis of long-term dosing of cannabidiol in humans, the terminal elimination half-life is estimated to be >134 h.[71] Cannabidiol is metabolized in the liver as well as in the intestines by cytochrome P450 enzymes.[4][68]
At room temperature, cannabidiol is a colorless crystalline solid.[74] In strongly basic media and the presence of air, it is oxidized to cannabinodiol (CBND) and a quinone called HU-331.[75] Under acidic conditions it cyclizes to THC,[76] which also occurs during pyrolysis,[77] and during smoking.[28][78] The synthesis of cannabidiol has been accomplished by several research groups.[79][80][81]

| Category | Compound | Description |
|---|---|---|
| Analog | 4'-Fluorocannabidiol | fluorinated cannabidiol derivative of CBD |
| Analog | Cannabidibutol (CBDB) | an analogue to tetrahydrocannabutol (THCB) |
| Analog | Cannabidiol diacetate | a semi-synthetic derivative of CBD |
| Analog | Cannabidiol dimethyl ether (CBDD) | an analog of CBD |
| Analog | H4-CBD | a structural analog of CBD |
| Analog | HU-320 | a structural analog of CBD |
| Analog | KLS-13019 | a structural analog of CBD |
| Homologue | 8,9-Dihydrocannabidiol (H2CBD) | synthetic derivative of CBD |
| Homologue | Cannabidiorcol (CBD-C1) | a homologue of CBD |
| Homologue | Cannabidiphorol (CBDP) | the heptyl-homologue of CBD |
| Homologue | Cannabidivarin (CBDV) | a homologue of CBD |
| Homologue | CBD-DMH | synthetic homologue of CBD |
| Homologue | Δ-6-Cannabidiol | a positional isomer of CBD |
| Isomer | Abnormal cannabidiol | a synthetic regioisomer of CBD |
| Metabolite | 7-Hydroxycannabidiol (7-OH-CBD) | an active metabolite of CBD |
| Precursor | Cannabidiolic acid (CBDA) | precursor to CBD |

Cannabis produces CBD through the same metabolic pathway as THC, until the next to last step, where CBDA synthase performs catalysis instead of THCA synthase.[84]

| Formal numbering | Terpenoid numbering | Number of stereoisomers | Natural occurrence | Convention on Psychotropic Substances Schedule | Structure | |||
|---|---|---|---|---|---|---|---|---|
| Short name | Chiral centers | Full name | Short name | Chiral centers | ||||
| Δ5-Cannabidiol | 1 and 3 | 2-(6-isopropenyl-3-methyl-5-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ4-Cannabidiol | 1 and 3 | 4 | No | Unscheduled | 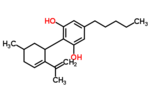 |
| Δ4-Cannabidiol | 1, 3 and 6 | 2-(6-isopropenyl-3-methyl-4-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ5-Cannabidiol | 1, 3 and 4 | 8 | No | Unscheduled |  |
| Δ3-Cannabidiol | 1 and 6 | 2-(6-isopropenyl-3-methyl-3-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ6-Cannabidiol | 3 and 4 | 4 | Yes | Unscheduled |  |
| Δ3,7-Cannabidiol | 1 and 6 | 2-(6-isopropenyl-3-methylenecyclohex-1-yl)-5-pentyl-1,3-benzenediol | Δ1,7-Cannabidiol | 3 and 4 | 4 | No | Unscheduled | 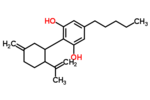 |
| Δ2-Cannabidiol | 1 and 6 | 2-(6-isopropenyl-3-methyl-2-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ1-Cannabidiol | 3 and 4 | 4 | Yes | Unscheduled |  |
| Δ1-Cannabidiol | 3 and 6 | 2-(6-isopropenyl-3-methyl-1-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ2-Cannabidiol | 1 and 4 | 4 | No | Unscheduled | 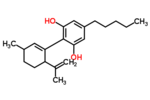 |
| Δ6-Cannabidiol | 3 | 2-(6-isopropenyl-3-methyl-6-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ3-Cannabidiol | 1 | 2 | No | Unscheduled | 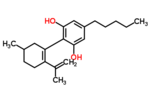 |
In the typical operating temperature range of e-cigarettes (250–400 °C (482–752 °F)), 25–52% of CBD is transformed into other chemical substances: Δ9-THC, Δ8-THC, cannabinol and cannabichromene as predominant pyrolysates. From a chemical point of view, CBD in e-cigarettes can be considered a precursor of THC.[28]
Numerous synthetic derivatives of CBD are known, and have been researched for generally similar applications as CBD itself.[85]
Efforts to isolate the active ingredients in cannabis were made in the 19th century.[86] Cannabidiol was studied in 1940 from Minnesota wild hemp[86] and Egyptian Cannabis indica resin.[87][88] The chemical formula of CBD was proposed from a method for isolating it from wild hemp.[86] Its structure and stereochemistry were determined in 1963.[89]
Selective breeding of cannabis plants has expanded and diversified as commercial and therapeutic markets develop. Some growers in the US succeeded in lowering the proportion of CBD-to-THC to accommodate customers who preferred varietals that were more mind-altering due to the higher THC and lower CBD content.[90] In the US, hemp is classified by the federal government as cannabis containing no more than 0.3% THC by dry weight. This classification was established in the 2018 Farm Bill and was refined to include hemp-sourced extracts, cannabinoids, and derivatives in the definition of hemp.[91]
Cannabidiol is the generic name of the drug and its INN.[92]

Food and beverage products containing cannabidiol were widely marketed in the United States as early as 2017.[93] Hemp seed ingredients which do not naturally contain THC or CBD (but which may be contaminated with trace amounts on the outside during harvesting) were declared by the US Food and Drug Administration as generally recognized as safe (GRAS) in December 2018. CBD itself has not been declared GRAS, and under US federal law is illegal to sell as a food, dietary supplement, or animal feed.[53] State laws vary considerably as non-medical cannabis and derived products have been legalized in various jurisdictions.[94] Despite once having a promising market, the industry for CBD stalled out during the COVID-19 pandemic beginning in 2020, and, by 2024, it collapsed due to withdrawal of investors, the absence of a FDA ruling on efficacy and safety, inconsistent state-by-state legislation, and consumer ambivalence.[94]
Similar to energy drinks and protein bars which may contain vitamin or herbal additives, food and beverage items can be infused with CBD as an alternative means of ingesting the substance.[94] In the United States, numerous products are marketed as containing CBD, but in reality contain little or none.[53][95] Some companies marketing CBD-infused food products with claims that are similar to the effects of prescription drugs have received warning letters from the FDA for making unsubstantiated health claims.[53][96] In February 2019, the New York City Department of Health announced plans to fine restaurants that sell food or drinks containing CBD, beginning in October 2019.[97]
Studies conducted by the FDA from 2014 through 2019 have determined that a majority of CBD products are not accurately labeled with the amount of CBD they contain.[98] For example, a 2017 analysis of cannabidiol content in oil, tincture, or liquid vape products purchased online in the United States showed that 69% were mislabeled, with 43% having higher and 26% having lower content than stated on product labels.[99][56] In 2020, the FDA conducted a study of 147 CBD products and found that half contained THC.[98][100]
From 2015 to November 2022, the FDA issued dozens of warning letters to American manufacturers of CBD products for false advertising and illegal interstate marketing of CBD as an unapproved drug to treat diseases, such as cancer, osteoarthritis, symptoms of opioid withdrawal, Alzheimer's disease, and pet disorders.[57] Chemical analysis of CBD products found that many did not contain the levels of CBD claimed in advertising.[57]
In December 2020, the Federal Trade Commission initiated a law enforcement crackdown on American companies marketing CBD products as unapproved drugs.[101][102] The warning also applied to hemp CBD capsules and oil that were being marketed illegally while not adhering to the federal definition of a dietary supplement.[102]
Cannabidiol has been used by professional and amateur athletes across disciplines and countries, with the World Anti-Doping Agency removing CBD from its banned substances list. The United States Anti-Doping Agency and United Kingdom-Anti-Doping Agency do not have anti-CBD policies, with the latter stating that, "CBD is not currently listed on the World Anti-Doping Agency Prohibited List. As a result, it is permitted to use in sport, though the intended benefits are unclear and not backed by clinical evidence. All other cannabinoids (including but not limited to cannabis, hashish, marijuana, and THC) are prohibited in-competition. The intention of the regulations is to prohibit cannabinoids that activate the same receptors in the brain as activated by THC."[103][104]
In 2019, the cannabis manufacturer Canopy Growth acquired majority ownership of BioSteel Sports Nutrition, which is developing CBD products under endorsement by numerous professional athletes.[105] The National Hockey League Alumni Association began a project with Canopy Growth to determine if CBD or other cannabis products might improve neurological symptoms and quality of life in head-injured players.[105] Some sports leagues have announced sponsorships with CBD companies, such as Major League Baseball (Charlotte's Web) and Ultimate Fighting Championship (Love Hemp).[106][107] Numerous professional athletes use CBD, primarily for treating pain.[105][108][109]
Prescription medicine (Schedule 4) for therapeutic use containing two percent (2.0%) or less of other cannabinoids commonly found in cannabis (such as ∆9-THC). A Schedule 4 drug under the SUSMP is a Prescription Only Medicine, or Prescription Animal Remedy – Substances, the use or supply of which should be by or on the order of persons permitted by state or territory legislation to prescribe and should be available from a pharmacist on prescription.[110]
In June 2020, the Australian Therapeutic Goods Administration (TGA) published a consultation on a proposal to pave the way to make "low dose" CBD available to consumer/patients via pharmacists only through moving products from Schedule 4 to 3.[111] Any products sold would need to have their safety, quality and efficacy pre-assessed by the TGA and be formally approved for sale (details to be outlined by TGA). They would be made available to over 18s only, with the maximum daily dose of 60 mg/day, up to 2% THC finished product allowed, 30-day maximum supply, plant-derived or synthetic. This proposal is based on an initial literature review on the safety of low dose CBD published by the TGA in April 2020.[112] Epidyolex was approved for the adjunctive therapy of seizures associated with Lennox–Gastaut syndrome or with Dravet syndrome in September 2020.[2]
In 2020, Bulgaria became the first country in the European Union to allow retail sales of food products and supplements containing CBD, despite the ongoing discussion within the EU about the classification of CBD as a novel food.[113] However, there exists a legal gap because of the lack of a legally-permissible minimum amount of THC in the products containing cannabinoids.[114]
In October 2018, cannabidiol became legal for recreational and medical use by the federal Cannabis Act.[115][116][117] As of August 2019[update], CBD products in Canada could only be sold by authorized retailers or federally licensed medical companies, limiting their access to the general public.[118] Nonetheless, with online delivery services and over 2,600 authorized cannabis retail stores as of October 2021[update], accessibility has steadily increased over time.[119][120] The Canadian government states that CBD products "are subject to all of the rules and requirements that apply to cannabis under the Cannabis Act and its regulations."[115] It requires "a processing licence to manufacture products containing CBD for sale, no matter what the source of the CBD is, and that CBD and products containing CBD, such as cannabis oil, may only be sold by an authorized retailer or licensed seller of medical CBD."[115] Edible CBD products were scheduled to be permitted for sale in Canada on October 17, 2019, for human consumption.[115]
As of August 2020[update], it was still illegal to carry cannabis and cannabis-derived products (including products containing CBD) across the Canadian border. If one carries any amount of cannabis for any purpose (including medical), it needs to be declared to the Canada Border Services Agency. Not declaring it is a serious criminal offence.[121]
As of May 2023, the State Agricultural and Food Inspection of the Czech Republic is putting together broad regulations regarding a ban on CBD products.[122] They will make it illegal to sell products containing cannabidiol and other cannabinoids derived from hemp, as a result of EU Novel Food Regulation. In case of Czech Republic, European Industrial Hemp Association has submitted an official request to the Czech Republic to recognize natural hemp extracts with cannabinoids as traditional food.[123]

In 2019, the European Commission announced that CBD and other cannabinoids would be classified as "novel foods",[124] meaning that CBD products would require authorization under the EU Novel Food Regulation stating that because "this product was not used as a food or food ingredient before May 15, 1997, before it may be placed on the market in the EU as a food or food ingredient, a safety assessment under the Novel Food Regulation is required."[125] The recommendation – applying to CBD extracts, synthesized CBD, and all CBD products, including CBD oil – was scheduled for a final ruling by the European Commission in March 2019.[124] If approved, manufacturers of CBD products would be required to conduct safety tests and prove safe consumption, indicating that CBD products would not be eligible for legal commerce until at least 2021.[124] In December 2020, the European Commission concluded that CBD should not be considered as drug and can be qualified as food.[126]
Cannabidiol is listed in the EU Cosmetics Ingredient Database (CosIng).[127] However, the listing of an ingredient, assigned with an INCI name, in CosIng does not mean it is to be used in cosmetic products or is approved for such use.[127]
Several industrial hemp varieties can be legally cultivated in Western Europe. A variety such as "Fedora 17" has a cannabinoid profile consistently around 1%, with THC less than 0.3%.[128]
In 2022, the HKSAR Government proposed a ban on any use of cannabidiol (including for academic research and by medical professionals) within the Hong Kong territory, making Hong Kong the first jurisdiction in the world to have complete prohibition of cannabidiol, starting from February 1, 2023,[129] in part due to the possible presence of THC which is illegal in Hong Kong, according to a research subsidized by the Hong Kong SAR Government.[130][131][132]
In 2017, the New Zealand government made changes to the regulations so that restrictions would be removed, which meant a doctor was able to prescribe cannabidiol to patients.[133]
The passing of the Misuse of Drugs (Medicinal Cannabis) Amendment Act in December 2018 means cannabidiol is no longer a controlled drug in New Zealand, but is a prescription medicine under the Medicines Act, with the restriction that "the tetrahydrocannabinols (THCs) and specified substances within the product must not exceed 2 percent of the total CBD, tetrahydrocannabinol (THC) and other specified substances."[134]
According to a document received in response to an appeal to the Ministry of Internal Affairs of the Russian Federation, measures of state control in the Russian Federation regarding CBD have not been established. However, there is also a response from the Ministry of Health of the Russian Federation indicating that CBD can be considered as an isomer of restricted THC. The "isomer" argument is nonetheless vague, as progesterone, which is freely sold in pharmacies, is also an isomer of THC, all three being C
21H
30O
2.[135] On February 17, 2020, the deputy of the Moscow City Duma Darya Besedina sent an official request to the Prime Minister of the Russian Federation Mikhail Mishustin with a request to eliminate that legal ambiguity by publishing official explanations and, if necessary, making required changes in the corresponding government decree.[136]
Singapore allows medical cannabis on a case-by-case basis, usually as a last resort drug. Each case is evaluated by the government, and largely comes in the form of Cannabidiol. However, the country is flexible to what is required for patient treatment, despite having some of the strictest drug laws in the world.
Cannabidiol is classified as a medical product in Sweden.[137] However, in July 2019, Supreme Court of Sweden ruled that CBD oil with any concentration of THC falls under the narcotic control laws.[138]
While THC remains illegal, cannabidiol is not subject to the Swiss Narcotic Acts because it does not produce a comparable psychoactive effect.[139] Cannabis products containing less than 1% THC can be sold and purchased legally.[140][141]
On April 7, 2021, the Ukrainian government legalised use of isolated cannabidiol. Additionally, it approved Nabiximols, a cannabidiol-containing drug, for medical use.[142]
Cannabidiol, in an oral-mucosal spray formulation combined with delta-9-tetrahydrocannabinol, is a product available by prescription for the relief of severe spasticity due to multiple sclerosis (where other anti-spasmodics have not been effective) in the United Kingdom.[143]
Until 2017, products containing cannabidiol marketed for medical purposes were classed as medicines by the UK regulatory body, the Medicines and Healthcare products Regulatory Agency (MHRA), and could not be marketed without regulatory approval for the medical claims.[144][145] As of 2018[update], cannabis oil is legal to possess, buy, and sell in the UK, providing the product does not contain more than 1 milligram of THC and is not advertised as providing a medicinal benefit.[7] Individual police officers and others who are ill-informed of the exact legislature pertaining to cannabidiol, however, may erroneously consider it of dubious legality, reflecting lack of awareness.[146]
In January 2019, the UK Food Standards Agency indicated it would regard CBD products, including CBD oil, as a novel food having no history of use before May 1997, and stated that such products must have authorisation and proven safety before being marketed.[124][147] The deadline for companies with existing products to submit a full and validated novel foods application with the FSA was March 31, 2021; failure to do so before this date would exclude those companies from selling CBD.[148] New products containing CBD after this deadline would require a fully approved application.[149]
In February 2020, the UK FSA advised vulnerable people, such as pregnant women, breastfeeding mothers, and those already taking medication for other medical concerns not to take CBD. The FSA further recommended that healthy adults should not consume more than 70 mg CBD per day.[148]
Cannabidiol is scheduled under the Single Convention on Narcotic Drugs as cannabis. International Narcotics Control Board reminds Member States that, at the reconvened sixty-third session of the Commission on Narcotic Drugs, in December 2020, the States members of the Commission rejected the recommendation of WHO that a footnote be added to the entry for cannabis and cannabis resin in Schedule I of the 1961 Convention as amended to exempt from international control preparations containing predominantly CBD and not more than 0.2 per cent of delta-9-THC.[150]
As of 2023[update], cannabidiol extracted from marijuana remains a Schedule I Controlled Substance,[53][151][152] and is not approved as a prescription drug or dietary supplement or allowed for interstate commerce in the United States.[52] CBD derived from hemp (with 0.3% THC or lower) is legal to sell as a cosmetics ingredient or for other purposes not regulated by the FDA, but cannot be sold under federal law as an ingredient in food, dietary supplement, or animal feed.[53][153] It is a common misconception that the legal ability to sell hemp (which may contain CBD), and hemp extracts and derivatives (including CBD), makes CBD legal for sale as a supplement or medicine.[153][154]
In September 2018, the GW Pharmaceuticals drug Epidiolex was placed in Schedule V of the Controlled Substances Act by the Drug Enforcement Administration (DEA),[155] following its approval by the FDA for rare types of childhood epilepsy.[4] It was then removed from the Controlled Substances Act by the DEA in April 2020.[156] Epidiolex is available for prescription use in all 50 states.[157]
In 2013, a CNN program that featured Charlotte's Web cannabis brought increased attention to the use of CBD for the treatment of seizure disorders in children.[158][159] A number of states passed laws over the next few years to allow the use of low-THC, high-CBD cannabis oil in such situations.[160] These states were in addition to the states that had already legalized cannabis for medical or recreational use.[160] Many states further relaxed their laws regarding CBD following the passage of the 2018 Farm Bill.[161][162]
The 2014 Farm Bill[163] legalized the sale of "non-viable hemp material" grown within states participating in the Hemp Pilot Program which defined hemp as cannabis containing less than 0.3% of THC.[164] The 2018 Farm Bill removed the hemp plant and all "derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers, whether growing or not, with a delta-9 tetrahydrocannabinol concentration of not more than 0.3 percent on a dry weight basis," including CBD, from the Controlled Substances Act, making them legal to manufacture in the United States.[29][165][166] The FDA retains regulatory authority over hemp-derived CBD,[154] while the DEA is not involved in the regulation of legally-compliant hemp and hemp products.[167] The 2018 Farm Bill requires that research and development of CBD for a therapeutic purpose would have to be conducted under notification and reporting to the FDA.[154][54]
The number of research projects and scientific publications on cannabidiol and other cannabinoids in pets surged in the late 2010s; nonetheless, as of December 2020[update], there were no hemp-derived, cannabinoid-rich registered veterinary medicinal products in any of the major regions (see #Legal status).
In the US and other territories there are, however, numerous veterinary nutraceutical products available over the counter (OTC). The lack of clarity in the regulations governing veterinary hemp food supplements allows for products of questionable quality to flood the market,[168][169] which may pose a risk to the wellbeing of pets and owners.
To understand better the benefits of CBD and associated compounds for the quality of life of animals, companies specialized in CBD products for animals have been funding research projects.[170][171][172][173]
CBD's ability to help regulate the endocannabinoid system[174][175][176] and reduce the release of excitatory neurotransmitters could result in a retrograde inhibitory signal that lessens chronic pain responses. Studies in dogs with chronic pain associated with osteoarthritis showed an increase in level of activity in animals receiving CBD-rich food supplements.[177][178][179][171]
From the results seen in humans with drugs such as Epidiolex and Sativex in scientific studies and reviews,[180] it could be expected that CBD-based products would be helpful to manage seizures in dogs. However, despite the numerous case reports presented by veterinary neurologists supporting the benefits of CBD as adjunctive therapy, as of December 2020[update], published controlled studies have not shown a statistically significant decrease in the number of seizures across the groups receiving CBD.[170][172]
The oral bioavailability of CBD varies greatly across species and it is linked to the presentation and the time of administration.[181][173][182] A 24-hour kinetic examination in dogs showed that the absorption of the cannabidiolic acid (CBDA) does occur, and that this molecule is absorbed least twice as well as CBD post oral ingestion.[181][173][183]
It was found that the major metabolites of CBD in humans (7-OH-CBD and 7-COOH-CBD) are not prevalent in dogs, while 6-OH-CBD was found to be the primary metabolite in dogs receiving a CBD-enriched cannabis-derived herbal extract,[184] suggesting that canine and human CBD metabolic route might be somewhat different.[182]
As of 2022[update], little evidence exists for cannabidiol reducing psychiatric disorders in humans.[17][18][19][36][185]
In the United States, federal illegality complicates conducting historic research on CBD.[186]
These companies are selling CBD containing products that people may confuse for traditional foods or beverages which may result in unintentional consumption or overconsumption of CBD. CBD-containing products in forms that are appealing to children, such as gummies, hard candies and cookies, are especially concerning.
BioSteel's brand ambassadors also include well-known athletes across major sports leagues in North America, which could be beneficial as the company's attempt to push regulated CBD nutrition products into the mainstream health and wellness segments
Unlike drugs approved by the FDA, the manufacturing process of these products has not been subject to FDA review as part of the drug approval process, and there has been no FDA evaluation of whether these products are effective for their intended use, what the proper dosage is, how they could interact with FDA-approved drugs, or whether they have dangerous side effects or other safety concerns.
| Cannabis
Temporal range: Early Miocene – Present
|
|
|---|---|
 |
|
| Common hemp | |
| Scientific classification |
|
| Kingdom: | Plantae |
| Clade: | Tracheophytes |
| Clade: | Angiosperms |
| Clade: | Eudicots |
| Clade: | Rosids |
| Order: | Rosales |
| Family: | Cannabaceae |
| Genus: | Cannabis L. |
| Species[1] | |
|
|
| Part of a series on |
| Cannabis |
|---|
 |
Cannabis (/ˈkænÉ™bɪs/ ⓘ)[2] is a genus of flowering plants in the family Cannabaceae that is widely accepted as being indigenous to and originating from the continent of Asia.[3][4][5] However, the number of species is disputed, with as many as three species being recognized: Cannabis sativa, C. indica, and C. ruderalis. Alternatively, C. ruderalis may be included within C. sativa, or all three may be treated as subspecies of C. sativa,[1][6][7][8] or C. sativa may be accepted as a single undivided species.[9]
The plant is also known as hemp, although this term is usually used to refer only to varieties cultivated for non-drug use. Hemp has long been used for fibre, seeds and their oils, leaves for use as vegetables, and juice. Industrial hemp textile products are made from cannabis plants selected to produce an abundance of fibre.
Cannabis also has a long history of being used for medicinal purposes, and as a recreational drug known by several slang terms, such as marijuana, pot or weed. Various cannabis strains have been bred, often selectively to produce high or low levels of tetrahydrocannabinol (THC), a cannabinoid and the plant's principal psychoactive constituent. Compounds such as hashish and hash oil are extracted from the plant.[10] More recently, there has been interest in other cannabinoids like cannabidiol (CBD), cannabigerol (CBG), and cannabinol (CBN).
Cannabis is a Scythian word.[11][12][13] The ancient Greeks learned of the use of cannabis by observing Scythian funerals, during which cannabis was consumed.[12] In Akkadian, cannabis was known as qunubu (ðŽ¯ðŽ«ðŽ ðŽð‚).[12] The word was adopted in to the Hebrew language as qaneh bosem (×§Ö¸× Ö¶×” בֹּשׂ×).[12]


Cannabis is an annual, dioecious, flowering herb. The leaves are palmately compound or digitate, with serrate leaflets.[14] The first pair of leaves usually have a single leaflet, the number gradually increasing up to a maximum of about thirteen leaflets per leaf (usually seven or nine), depending on variety and growing conditions. At the top of a flowering plant, this number again diminishes to a single leaflet per leaf. The lower leaf pairs usually occur in an opposite leaf arrangement and the upper leaf pairs in an alternate arrangement on the main stem of a mature plant.
The leaves have a peculiar and diagnostic venation pattern (which varies slightly among varieties) that allows for easy identification of Cannabis leaves from unrelated species with similar leaves. As is common in serrated leaves, each serration has a central vein extending to its tip, but in Cannabis this originates from lower down the central vein of the leaflet, typically opposite to the position of the second notch down. This means that on its way from the midrib of the leaflet to the point of the serration, the vein serving the tip of the serration passes close by the intervening notch. Sometimes the vein will pass tangentially to the notch, but often will pass by at a small distance; when the latter happens a spur vein (or occasionally two) branches off and joins the leaf margin at the deepest point of the notch. Tiny samples of Cannabis also can be identified with precision by microscopic examination of leaf cells and similar features, requiring special equipment and expertise.[15]
All known strains of Cannabis are wind-pollinated[16] and the fruit is an achene.[17] Most strains of Cannabis are short day plants,[16] with the possible exception of C. sativa subsp. sativa var. spontanea (= C. ruderalis), which is commonly described as "auto-flowering" and may be day-neutral.
Cannabis is predominantly dioecious,[16][18] having imperfect flowers, with staminate "male" and pistillate "female" flowers occurring on separate plants.[19] "At a very early period the Chinese recognized the Cannabis plant as dioecious",[20] and the (c. 3rd century BCE) Erya dictionary defined xi 枲 "male Cannabis" and fu 莩 (or ju 苴) "female Cannabis".[21] Male flowers are normally borne on loose panicles, and female flowers are borne on racemes.[22]
Many monoecious varieties have also been described,[23] in which individual plants bear both male and female flowers.[24] (Although monoecious plants are often referred to as "hermaphrodites", true hermaphrodites – which are less common in Cannabis – bear staminate and pistillate structures together on individual flowers, whereas monoecious plants bear male and female flowers at different locations on the same plant.) Subdioecy (the occurrence of monoecious individuals and dioecious individuals within the same population) is widespread.[25][26][27] Many populations have been described as sexually labile.[28][29][30]
As a result of intensive selection in cultivation, Cannabis exhibits many sexual phenotypes that can be described in terms of the ratio of female to male flowers occurring in the individual, or typical in the cultivar.[31] Dioecious varieties are preferred for drug production, where the fruits (produced by female flowers) are used. Dioecious varieties are also preferred for textile fiber production, whereas monoecious varieties are preferred for pulp and paper production. It has been suggested that the presence of monoecy can be used to differentiate licit crops of monoecious hemp from illicit drug crops,[25] but sativa strains often produce monoecious individuals, which is possibly as a result of inbreeding.


Cannabis has been described as having one of the most complicated mechanisms of sex determination among the dioecious plants.[31] Many models have been proposed to explain sex determination in Cannabis.
Based on studies of sex reversal in hemp, it was first reported by K. Hirata in 1924 that an XY sex-determination system is present.[29] At the time, the XY system was the only known system of sex determination. The X:A system was first described in Drosophila spp in 1925.[32] Soon thereafter, Schaffner disputed Hirata's interpretation,[33] and published results from his own studies of sex reversal in hemp, concluding that an X:A system was in use and that furthermore sex was strongly influenced by environmental conditions.[30]
Since then, many different types of sex determination systems have been discovered, particularly in plants.[18] Dioecy is relatively uncommon in the plant kingdom, and a very low percentage of dioecious plant species have been determined to use the XY system. In most cases where the XY system is found it is believed to have evolved recently and independently.[34]
Since the 1920s, a number of sex determination models have been proposed for Cannabis. Ainsworth describes sex determination in the genus as using "an X/autosome dosage type".[18]
The question of whether heteromorphic sex chromosomes are indeed present is most conveniently answered if such chromosomes were clearly visible in a karyotype. Cannabis was one of the first plant species to be karyotyped; however, this was in a period when karyotype preparation was primitive by modern standards. Heteromorphic sex chromosomes were reported to occur in staminate individuals of dioecious "Kentucky" hemp, but were not found in pistillate individuals of the same variety. Dioecious "Kentucky" hemp was assumed to use an XY mechanism. Heterosomes were not observed in analyzed individuals of monoecious "Kentucky" hemp, nor in an unidentified German cultivar. These varieties were assumed to have sex chromosome composition XX.[35] According to other researchers, no modern karyotype of Cannabis had been published as of 1996.[36] Proponents of the XY system state that Y chromosome is slightly larger than the X, but difficult to differentiate cytologically.[37]
More recently, Sakamoto and various co-authors[38][39] have used random amplification of polymorphic DNA (RAPD) to isolate several genetic marker sequences that they name Male-Associated DNA in Cannabis (MADC), and which they interpret as indirect evidence of a male chromosome. Several other research groups have reported identification of male-associated markers using RAPD and amplified fragment length polymorphism.[40][28][41] Ainsworth commented on these findings, stating,
It is not surprising that male-associated markers are relatively abundant. In dioecious plants where sex chromosomes have not been identified, markers for maleness indicate either the presence of sex chromosomes which have not been distinguished by cytological methods or that the marker is tightly linked to a gene involved in sex determination.[18]
Environmental sex determination is known to occur in a variety of species.[42] Many researchers have suggested that sex in Cannabis is determined or strongly influenced by environmental factors.[30] Ainsworth reviews that treatment with auxin and ethylene have feminizing effects, and that treatment with cytokinins and gibberellins have masculinizing effects.[18] It has been reported that sex can be reversed in Cannabis using chemical treatment.[43] A polymerase chain reaction-based method for the detection of female-associated DNA polymorphisms by genotyping has been developed.[44]
Cannabis plants produce a large number of chemicals as part of their defense against herbivory. One group of these is called cannabinoids, which induce mental and physical effects when consumed.
Cannabinoids, terpenes, terpenoids, and other compounds are secreted by glandular trichomes that occur most abundantly on the floral calyxes and bracts of female plants.[46]
Cannabis, like many organisms, is diploid, having a chromosome complement of 2n=20, although polyploid individuals have been artificially produced.[47] The first genome sequence of Cannabis, which is estimated to be 820 Mb in size, was published in 2011 by a team of Canadian scientists.[48]

The genus Cannabis was formerly placed in the nettle family (Urticaceae) or mulberry family (Moraceae), and later, along with the genus Humulus (hops), in a separate family, the hemp family (Cannabaceae sensu stricto).[49] Recent phylogenetic studies based on cpDNA restriction site analysis and gene sequencing strongly suggest that the Cannabaceae sensu stricto arose from within the former family Celtidaceae, and that the two families should be merged to form a single monophyletic family, the Cannabaceae sensu lato.[50][51]
Various types of Cannabis have been described, and variously classified as species, subspecies, or varieties:[52]
Cannabis plants produce a unique family of terpeno-phenolic compounds called cannabinoids, some of which produce the "high" which may be experienced from consuming marijuana. There are 483 identifiable chemical constituents known to exist in the cannabis plant,[53] and at least 85 different cannabinoids have been isolated from the plant.[54] The two cannabinoids usually produced in greatest abundance are cannabidiol (CBD) and/or Δ9-tetrahydrocannabinol (THC), but only THC is psychoactive.[55] Since the early 1970s, Cannabis plants have been categorized by their chemical phenotype or "chemotype", based on the overall amount of THC produced, and on the ratio of THC to CBD.[56] Although overall cannabinoid production is influenced by environmental factors, the THC/CBD ratio is genetically determined and remains fixed throughout the life of a plant.[40] Non-drug plants produce relatively low levels of THC and high levels of CBD, while drug plants produce high levels of THC and low levels of CBD. When plants of these two chemotypes cross-pollinate, the plants in the first filial (F1) generation have an intermediate chemotype and produce intermediate amounts of CBD and THC. Female plants of this chemotype may produce enough THC to be utilized for drug production.[56][57]

Whether the drug and non-drug, cultivated and wild types of Cannabis constitute a single, highly variable species, or the genus is polytypic with more than one species, has been a subject of debate for well over two centuries. This is a contentious issue because there is no universally accepted definition of a species.[58] One widely applied criterion for species recognition is that species are "groups of actually or potentially interbreeding natural populations which are reproductively isolated from other such groups."[59] Populations that are physiologically capable of interbreeding, but morphologically or genetically divergent and isolated by geography or ecology, are sometimes considered to be separate species.[59] Physiological barriers to reproduction are not known to occur within Cannabis, and plants from widely divergent sources are interfertile.[47] However, physical barriers to gene exchange (such as the Himalayan mountain range) might have enabled Cannabis gene pools to diverge before the onset of human intervention, resulting in speciation.[60] It remains controversial whether sufficient morphological and genetic divergence occurs within the genus as a result of geographical or ecological isolation to justify recognition of more than one species.[61][62][63]
The genus Cannabis was first classified using the "modern" system of taxonomic nomenclature by Carl Linnaeus in 1753, who devised the system still in use for the naming of species.[64] He considered the genus to be monotypic, having just a single species that he named Cannabis sativa L.[a 1] Linnaeus was familiar with European hemp, which was widely cultivated at the time. This classification was supported by Christiaan Hendrik Persoon (in 1807), Lindley (in 1838) and De Candollee (in 1867). These first classification attempts resulted in a four group division:[65]
In 1785, evolutionary biologist Jean-Baptiste de Lamarck published a description of a second species of Cannabis, which he named Cannabis indica Lam.[66] Lamarck based his description of the newly named species on morphological aspects (trichomes, leaf shape) and geographic localization of plant specimens collected in India. He described C. indica as having poorer fiber quality than C. sativa, but greater utility as an inebriant. Also, C. indica was considered smaller, by Lamarck. Also, woodier stems, alternate ramifications of the branches, narrow leaflets, and a villous calyx in the female flowers were characteristics noted by the botanist.[65]
In 1843, William O’Shaughnessy, used "Indian hemp (C. indica)" in a work title. The author claimed that this choice wasn't based on a clear distinction between C. sativa and C. indica, but may have been influenced by the choice to use the term "Indian hemp" (linked to the plant's history in India), hence naming the species as indica.[65]
Additional Cannabis species were proposed in the 19th century, including strains from China and Vietnam (Indo-China) assigned the names Cannabis chinensis Delile, and Cannabis gigantea Delile ex Vilmorin.[67] However, many taxonomists found these putative species difficult to distinguish. In the early 20th century, the single-species concept (monotypic classification) was still widely accepted, except in the Soviet Union, where Cannabis continued to be the subject of active taxonomic study. The name Cannabis indica was listed in various Pharmacopoeias, and was widely used to designate Cannabis suitable for the manufacture of medicinal preparations.[68]

In 1924, Russian botanist D.E. Janichevsky concluded that ruderal Cannabis in central Russia is either a variety of C. sativa or a separate species, and proposed C. sativa L. var. ruderalis Janisch, and Cannabis ruderalis Janisch, as alternative names.[52] In 1929, renowned plant explorer Nikolai Vavilov assigned wild or feral populations of Cannabis in Afghanistan to C. indica Lam. var. kafiristanica Vav., and ruderal populations in Europe to C. sativa L. var. spontanea Vav.[57][67] Vavilov, in 1931, proposed a three species system, independently reinforced by Schultes et al (1975)[69] and Emboden (1974):[70] C. sativa, C. indica and C. ruderalis.[65]
In 1940, Russian botanists Serebriakova and Sizov proposed a complex poly-species classification in which they also recognized C. sativa and C. indica as separate species. Within C. sativa they recognized two subspecies: C. sativa L. subsp. culta Serebr. (consisting of cultivated plants), and C. sativa L. subsp. spontanea (Vav.) Serebr. (consisting of wild or feral plants). Serebriakova and Sizov split the two C. sativa subspecies into 13 varieties, including four distinct groups within subspecies culta. However, they did not divide C. indica into subspecies or varieties.[52][71][72] Zhukovski, in 1950, also proposed a two-species system, but with C. sativa L. and C. ruderalis.[73]
In the 1970s, the taxonomic classification of Cannabis took on added significance in North America. Laws prohibiting Cannabis in the United States and Canada specifically named products of C. sativa as prohibited materials. Enterprising attorneys for the defense in a few drug busts argued that the seized Cannabis material may not have been C. sativa, and was therefore not prohibited by law. Attorneys on both sides recruited botanists to provide expert testimony. Among those testifying for the prosecution was Dr. Ernest Small, while Dr. Richard E. Schultes and others testified for the defense. The botanists engaged in heated debate (outside of court), and both camps impugned the other's integrity.[61][62] The defense attorneys were not often successful in winning their case, because the intent of the law was clear.[74]

In 1976, Canadian botanist Ernest Small[75] and American taxonomist Arthur Cronquist published a taxonomic revision that recognizes a single species of Cannabis with two subspecies (hemp or drug; based on THC and CBD levels) and two varieties in each (domesticated or wild). The framework is thus:
This classification was based on several factors including interfertility, chromosome uniformity, chemotype, and numerical analysis of phenotypic characters.[56][67][76]
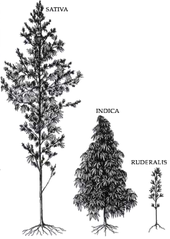
Professors William Emboden, Loran Anderson, and Harvard botanist Richard E. Schultes and coworkers also conducted taxonomic studies of Cannabis in the 1970s, and concluded that stable morphological differences exist that support recognition of at least three species, C. sativa, C. indica, and C. ruderalis.[77][78][79][80] For Schultes, this was a reversal of his previous interpretation that Cannabis is monotypic, with only a single species.[81] According to Schultes' and Anderson's descriptions, C. sativa is tall and laxly branched with relatively narrow leaflets, C. indica is shorter, conical in shape, and has relatively wide leaflets, and C. ruderalis is short, branchless, and grows wild in Central Asia. This taxonomic interpretation was embraced by Cannabis aficionados who commonly distinguish narrow-leafed "sativa" strains from wide-leafed "indica" strains.[82] McPartland's review finds the Schultes taxonomy inconsistent with prior work (protologs) and partly responsible for the popular usage.[83]
Molecular analytical techniques developed in the late 20th century are being applied to questions of taxonomic classification. This has resulted in many reclassifications based on evolutionary systematics. Several studies of random amplified polymorphic DNA (RAPD) and other types of genetic markers have been conducted on drug and fiber strains of Cannabis, primarily for plant breeding and forensic purposes.[84][85][28][86][87] Dutch Cannabis researcher E.P.M. de Meijer and coworkers described some of their RAPD studies as showing an "extremely high" degree of genetic polymorphism between and within populations, suggesting a high degree of potential variation for selection, even in heavily selected hemp cultivars.[40] They also commented that these analyses confirm the continuity of the Cannabis gene pool throughout the studied accessions, and provide further confirmation that the genus consists of a single species, although theirs was not a systematic study per se.
An investigation of genetic, morphological, and chemotaxonomic variation among 157 Cannabis accessions of known geographic origin, including fiber, drug, and feral populations showed cannabinoid variation in Cannabis germplasm. The patterns of cannabinoid variation support recognition of C. sativa and C. indica as separate species, but not C. ruderalis. C. sativa contains fiber and seed landraces, and feral populations, derived from Europe, Central Asia, and Turkey. Narrow-leaflet and wide-leaflet drug accessions, southern and eastern Asian hemp accessions, and feral Himalayan populations were assigned to C. indica.[57] In 2005, a genetic analysis of the same set of accessions led to a three-species classification, recognizing C. sativa, C. indica, and (tentatively) C. ruderalis.[60] Another paper in the series on chemotaxonomic variation in the terpenoid content of the essential oil of Cannabis revealed that several wide-leaflet drug strains in the collection had relatively high levels of certain sesquiterpene alcohols, including guaiol and isomers of eudesmol, that set them apart from the other putative taxa.[88]
A 2020 analysis of single-nucleotide polymorphisms reports five clusters of cannabis, roughly corresponding to hemps (including folk "Ruderalis") folk "Indica" and folk "Sativa".[89]
Despite advanced analytical techniques, much of the cannabis used recreationally is inaccurately classified. One laboratory at the University of British Columbia found that Jamaican Lamb's Bread, claimed to be 100% sativa, was in fact almost 100% indica (the opposite strain).[90] Legalization of cannabis in Canada (as of 17 October 2018[update]) may help spur private-sector research, especially in terms of diversification of strains. It should also improve classification accuracy for cannabis used recreationally. Legalization coupled with Canadian government (Health Canada) oversight of production and labelling will likely result in more—and more accurate—testing to determine exact strains and content. Furthermore, the rise of craft cannabis growers in Canada should ensure quality, experimentation/research, and diversification of strains among private-sector producers.[91]
The scientific debate regarding taxonomy has had little effect on the terminology in widespread use among cultivators and users of drug-type Cannabis. Cannabis aficionados recognize three distinct types based on such factors as morphology, native range, aroma, and subjective psychoactive characteristics. "Sativa" is the most widespread variety, which is usually tall, laxly branched, and found in warm lowland regions. "Indica" designates shorter, bushier plants adapted to cooler climates and highland environments. "Ruderalis" is the informal name for the short plants that grow wild in Europe and Central Asia.[83]
Mapping the morphological concepts to scientific names in the Small 1976 framework, "Sativa" generally refers to C. sativa subsp. indica var. indica, "Indica" generally refers to C. sativa subsp. i. kafiristanica (also known as afghanica), and "Ruderalis", being lower in THC, is the one that can fall into C. sativa subsp. sativa. The three names fit in Schultes's framework better, if one overlooks its inconsistencies with prior work.[83] Definitions of the three terms using factors other than morphology produces different, often conflicting results.
Breeders, seed companies, and cultivators of drug type Cannabis often describe the ancestry or gross phenotypic characteristics of cultivars by categorizing them as "pure indica", "mostly indica", "indica/sativa", "mostly sativa", or "pure sativa". These categories are highly arbitrary, however: one "AK-47" hybrid strain has received both "Best Sativa" and "Best Indica" awards.[83]
Cannabis likely split from its closest relative, Humulus (hops), during the mid Oligocene, around 27.8 million years ago according to molecular clock estimates. The centre of origin of Cannabis is likely in the northeastern Tibetan Plateau. The pollen of Humulus and Cannabis are very similar and difficult to distinguish. The oldest pollen thought to be from Cannabis is from Ningxia, China, on the boundary between the Tibetan Plateau and the Loess Plateau, dating to the early Miocene, around 19.6 million years ago. Cannabis was widely distributed over Asia by the Late Pleistocene. The oldest known Cannabis in South Asia dates to around 32,000 years ago.[92]
Cannabis is used for a wide variety of purposes.
According to genetic and archaeological evidence, cannabis was first domesticated about 12,000 years ago in East Asia during the early Neolithic period.[5] The use of cannabis as a mind-altering drug has been documented by archaeological finds in prehistoric societies in Eurasia and Africa.[93] The oldest written record of cannabis usage is the Greek historian Herodotus's reference to the central Eurasian Scythians taking cannabis steam baths.[94] His (c. 440 BCE) Histories records, "The Scythians, as I said, take some of this hemp-seed [presumably, flowers], and, creeping under the felt coverings, throw it upon the red-hot stones; immediately it smokes, and gives out such a vapour as no Greek vapour-bath can exceed; the Scyths, delighted, shout for joy."[95] Classical Greeks and Romans also used cannabis.
In China, the psychoactive properties of cannabis are described in the Shennong Bencaojing (3rd century AD).[96] Cannabis smoke was inhaled by Daoists, who burned it in incense burners.[96]
In the Middle East, use spread throughout the Islamic empire to North Africa. In 1545, cannabis spread to the western hemisphere where Spaniards imported it to Chile for its use as fiber. In North America, cannabis, in the form of hemp, was grown for use in rope, cloth and paper.[97][98][99][100]
Cannabinol (CBN) was the first compound to be isolated from cannabis extract in the late 1800s. Its structure and chemical synthesis were achieved by 1940, followed by some of the first preclinical research studies to determine the effects of individual cannabis-derived compounds in vivo.[101]
Globally, in 2013, 60,400 kilograms of cannabis were produced legally.[102]

Cannabis is a popular recreational drug around the world, only behind alcohol, caffeine, and tobacco. In the U.S. alone, it is believed that over 100 million Americans have tried cannabis, with 25 million Americans having used it within the past year.[when?][104] As a drug it usually comes in the form of dried marijuana, hashish, or various extracts collectively known as hashish oil.[10]
Normal cognition is restored after approximately three hours for larger doses via a smoking pipe, bong or vaporizer.[105] However, if a large amount is taken orally the effects may last much longer. After 24 hours to a few days, minuscule psychoactive effects may be felt, depending on dosage, frequency and tolerance to the drug.
Cannabidiol (CBD), which has no intoxicating effects by itself[55] (although sometimes showing a small stimulant effect, similar to caffeine),[106] is thought to attenuate (i.e., reduce)[107] the anxiety-inducing effects of high doses of THC, particularly if administered orally prior to THC exposure.[108]
According to Delphic analysis by British researchers in 2007, cannabis has a lower risk factor for dependence compared to both nicotine and alcohol.[109] However, everyday use of cannabis may be correlated with psychological withdrawal symptoms, such as irritability or insomnia,[105] and susceptibility to a panic attack may increase as levels of THC metabolites rise.[110][111] Cannabis withdrawal symptoms are typically mild and are not life-threatening.[112] Risk of adverse outcomes from cannabis use may be reduced by implementation of evidence-based education and intervention tools communicated to the public with practical regulation measures.[113]
In 2014 there were an estimated 182.5 million cannabis users worldwide (3.8% of the global population aged 15–64).[114] This percentage did not change significantly between 1998 and 2014.[114]
Medical cannabis (or medical marijuana) refers to the use of cannabis and its constituent cannabinoids, in an effort to treat disease or improve symptoms. Cannabis is used to reduce nausea and vomiting during chemotherapy, to improve appetite in people with HIV/AIDS, and to treat chronic pain and muscle spasms.[115][116] Cannabinoids are under preliminary research for their potential to affect stroke.[117] Evidence is lacking for depression, anxiety, attention deficit hyperactivity disorder, Tourette syndrome, post-traumatic stress disorder, and psychosis.[118] Two extracts of cannabis – dronabinol and nabilone – are approved by the FDA as medications in pill form for treating the side effects of chemotherapy and AIDS.[119]
Short-term use increases both minor and major adverse effects.[116] Common side effects include dizziness, feeling tired, vomiting, and hallucinations.[116] Long-term effects of cannabis are not clear.[120] Concerns including memory and cognition problems, risk of addiction, schizophrenia in young people, and the risk of children taking it by accident.[115]

The term hemp is used to name the durable soft fiber from the Cannabis plant stem (stalk). Cannabis sativa cultivars are used for fibers due to their long stems; Sativa varieties may grow more than six metres tall. However, hemp can refer to any industrial or foodstuff product that is not intended for use as a drug. Many countries regulate limits for psychoactive compound (THC) concentrations in products labeled as hemp.
Cannabis for industrial uses is valuable in tens of thousands of commercial products, especially as fibre[121] ranging from paper, cordage, construction material and textiles in general, to clothing. Hemp is stronger and longer-lasting than cotton. It also is a useful source of foodstuffs (hemp milk, hemp seed, hemp oil) and biofuels. Hemp has been used by many civilizations, from China to Europe (and later North America) during the last 12,000 years.[121][122] In modern times novel applications and improvements have been explored with modest commercial success.[123][124]
In the US, "industrial hemp" is classified by the federal government as cannabis containing no more than 0.3% THC by dry weight. This classification was established in the 2018 Farm Bill and was refined to include hemp-sourced extracts, cannabinoids, and derivatives in the definition of hemp.[125]


The Cannabis plant has a history of medicinal use dating back thousands of years across many cultures.[126] The Yanghai Tombs, a vast ancient cemetery (54 000 m2) situated in the Turfan district of the Xinjiang Uyghur Autonomous Region in northwest China, have revealed the 2700-year-old grave of a shaman. He is thought to have belonged to the Jushi culture recorded in the area centuries later in the Hanshu, Chap 96B.[127] Near the head and foot of the shaman was a large leather basket and wooden bowl filled with 789g of cannabis, superbly preserved by climatic and burial conditions. An international team demonstrated that this material contained THC. The cannabis was presumably employed by this culture as a medicinal or psychoactive agent, or an aid to divination. This is the oldest documentation of cannabis as a pharmacologically active agent.[128] The earliest evidence of cannabis smoking has been found in the 2,500-year-old tombs of Jirzankal Cemetery in the Pamir Mountains in Western China, where cannabis residue were found in burners with charred pebbles possibly used during funeral rituals.[129][130]
Settlements which date from c. 2200–1700 BCE in the Bactria and Margiana contained elaborate ritual structures with rooms containing everything needed for making drinks containing extracts from poppy (opium), hemp (cannabis), and ephedra (which contains ephedrine).[131]: 262  Although there is no evidence of ephedra being used by steppe tribes, they engaged in cultic use of hemp. Cultic use ranged from Romania to the Yenisei River and had begun by 3rd millennium BC Smoking hemp has been found at Pazyryk.[131]: 306 
Cannabis is first referred to in Hindu Vedas between 2000 and 1400 BCE, in the Atharvaveda. By the 10th century CE, it has been suggested that it was referred to by some in India as "food of the gods".[132] Cannabis use eventually became a ritual part of the Hindu festival of Holi. One of the earliest to use this plant in medical purposes was Korakkar, one of the 18 Siddhas.[133][134][self-published source?] The plant is called Korakkar Mooli in the Tamil language, meaning Korakkar's herb.[135][136]
In Buddhism, cannabis is generally regarded as an intoxicant and may be a hindrance to development of meditation and clear awareness. In ancient Germanic culture, Cannabis was associated with the Norse love goddess, Freya.[137][138] An anointing oil mentioned in Exodus is, by some translators, said to contain Cannabis.[139]
In modern times, the Rastafari movement has embraced Cannabis as a sacrament.[140] Elders of the Ethiopian Zion Coptic Church, a religious movement founded in the U.S. in 1975 with no ties to either Ethiopia or the Coptic Church, consider Cannabis to be the Eucharist, claiming it as an oral tradition from Ethiopia dating back to the time of Christ.[141] Like the Rastafari, some modern Gnostic Christian sects have asserted that Cannabis is the Tree of Life.[142][143] Other organized religions founded in the 20th century that treat Cannabis as a sacrament are the THC Ministry,[144] Cantheism,[145] the Cannabis Assembly[146] and the Church of Cognizance.
Since the 13th century CE, cannabis has been used among Sufis[147][148] – the mystical interpretation of Islam that exerts strong influence over local Muslim practices in Bangladesh, India, Indonesia, Turkey, and Pakistan. Cannabis preparations are frequently used at Sufi festivals in those countries.[147] Pakistan's Shrine of Lal Shahbaz Qalandar in Sindh province is particularly renowned for the widespread use of cannabis at the shrine's celebrations, especially its annual Urs festival and Thursday evening dhamaal sessions – or meditative dancing sessions.[149][150]
Cannabis is called kaneh bosem in Hebrew, which is now recognized as the Scythian word that Herodotus wrote as kánnabis (or cannabis).
Cannabis is a Scythian word (Benet 1975).
cite book: CS1 maint: location missing publisher (link)cite book: CS1 maint: location missing publisher (link)cite book: CS1 maint: location missing publisher (link)During the festival the air is heavy with drumbeats, chanting and cannabis smoke.